Rotational Spectroscopy Overview
Rotational spectroscopy is used to study the rotational states of molecules. Typically, the rotational constant
Key Formulae and Concepts
- Moment of Inertia:
where is the reduced mass and is the bond length. - Energy Levels: The energy of rotational states is given by
where is the rotational quantum number and is the rotational constant in wavenumbers. - Selection Rule: For microwave spectroscopy, the rotational transitions follow
, meaning the rotational quantum number changes by one unit.
Distribution of Populated Levels
- The distribution of molecules across different rotational levels follows the Boltzmann distribution. The most populated rotational level,
, is determined by the temperature and rotational constant:
Rotational Constant (Wavenumbers)
Rotational Constant (Frequency)
- The frequency equivalent of the rotational constant
is , where is in cm and is the speed of light in cm/s.
Microwave Absorption Spectroscopy
Microwave spectroscopy measures pure rotational transitions, typically for polar molecules with permanent dipole moments. The selection rule for microwave absorption is
-
Key Aspects:
- Narrow peak widths due to long lifetimes of excited states, as described by the Heisenberg uncertainty principle.
- Doppler broadening, a form of thermal doppler broadening, is observed because molecules move at different velocities, affecting the frequency recorded.
-
Spectral Spacing:
- The spacing between successive peaks is
or in frequency units. 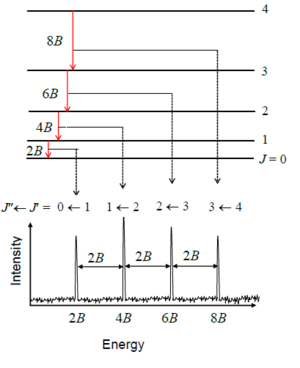
- The spacing between successive peaks is
Example Calculation:
Given a rotational constant
- Calculate the energy levels using
. - Find the most populated level,
, using the formula above.
Rotational Raman Spectroscopy
Raman spectroscopy probes rotational transitions, but with different selection rules:
- Stokes lines (energy gain):
- Anti-Stokes lines (energy loss):
- Lowest state:
, with lines appearing at
Selection Rules in Rotational Spectroscopy
- Microwave Spectroscopy:
- Requires a permanent dipole moment.
- Selection rule:
.
- Rotational Raman Spectroscopy:
- Can detect transitions in non-polar molecules.
- Selection rule:
.
Boltzmann Distribution
The relative population of molecules in a given rotational state
- The most populated rotational state is determined by both
and temperature, with calculated by the formula provided earlier.
Key Points in Spectroscopy
- Degeneracy: Each rotational level
has a degeneracy factor of . - Line Intensity: The intensity of each line is proportional to the square of the transition dipole moment. For a transition from
, the intensity is:
where
Thermal Doppler Broadening
- Arises due to the motion of molecules at different velocities.
- The Doppler effect shifts the frequency of the rotational transitions, and the degree of broadening is related to temperature.
Further Exploration
- You can watch this YouTube video for more in-depth understanding: https://youtu.be/qiTbuKE55Dk
- Wesley's video on rotational Raman spectroscopy: Wesley Video
References
- Peter Atkins Physical Chemistry QMC - Chapter 42 for detailed reading.