Mass Spectrometry Overview
How It Works
-
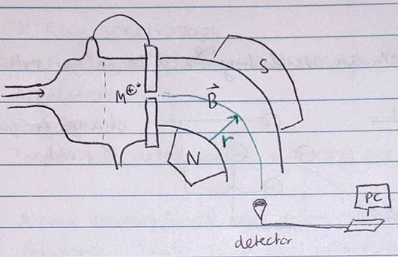
Old System and Basic Operation:
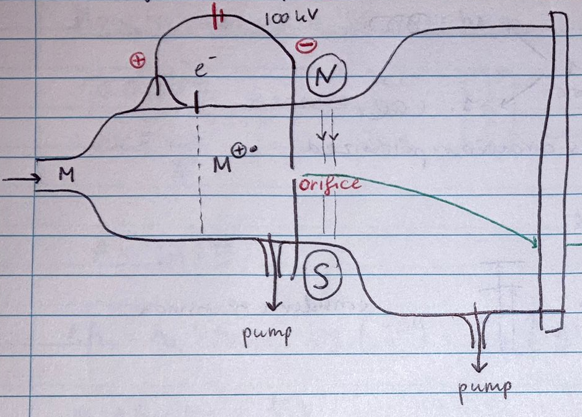
- A small droplet of sample is injected, and an electron is removed from the molecules, creating molecular radical cations.
- The application of a potential difference accelerates these molecular ion peaks through a slit towards a detection screen.
- A magnetic field deflects the course of the ion based on its mass, causing it to arrive at different locations on the phosphorous screen.
-
Theory:
- The mass-to-charge ratio
is given by , where: - (m) is the mass of the ion
- (z) is the charge of the ion
- (B) is the applied magnetic field
- (R) is the radius of deflection
- (V) is the applied voltage
- High vacuum conditions are required to prevent intermolecular energy exchanges.
- The resolution of the mass spectrometer is given by
. A resolution greater than 100,000 is considered High-Resolution Mass Spectrometry (HRMS).
- The mass-to-charge ratio
Detectors
-
Photomultiplier Tube (PMT):
- PMTs multiply the current produced by incident light, making detection possible.
- Electrons hit increasingly positive plates, resulting in a detectable current.
- This method replaces the old phosphorous screen.
-
Double Focusing Mass Spectrometer:
- Uses an electrostatic analyzer to refocus the spread of masses caused by flight, followed by a slit that further focuses this to the detector.
- This is used in HRMS and provides extremely high resolution.
-
Quadrupole Detector:
- A quadrupole detector measures mass using an oscillating field.
- Four voltage-carrying rods run the length of the flight path, creating complex oscillations in the flight path.
- Only specific
ratios oscillate such that they reach the detector, allowing for selective detection by adjusting the voltage. 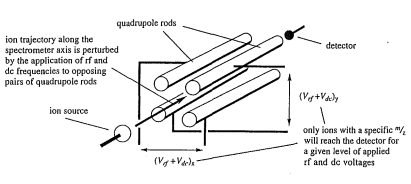
-
Time-of-Flight Mass Spectrometer (TOF-MS):
- TOF-MS is based on the principle that different mass particles with the same charge will fly at different speeds through a tube with an applied potential.
Ionization Techniques
-
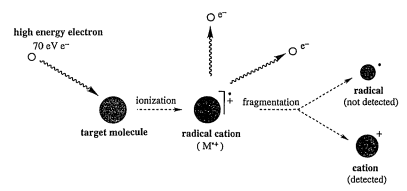
Electron Impact (EI) Ionization:
- In EI ionization, high-energy electrons impact the sample, causing the ejection of electrons and producing ionized fragments.
- This method can be too harsh, leading to over-fragmentation or decomposition.
-
Chemical Ionization (CI):
- CI uses an ionized gas to create quasi-molecular ions.
- The detected molecular ions are typically M+1 and M+RH, where R is the gas used.
-
Electrospray Ionization (ESI):
- ESI involves spraying molecules from a solution through a charged needle, creating a fine ionized mist where the solvent evaporates quickly.
- Fragmentation is typically not observed due to the mild conditions.
-
Matrix-Assisted Laser Desorption/Ionization (MALDI):
- MALDI ionizes a sample (often a protein) embedded in a matrix using laser light.
- It is often coupled with TOF-MS (MALDI-TOF).
Fragmentation Patterns
-
Predicting Fragmentation:
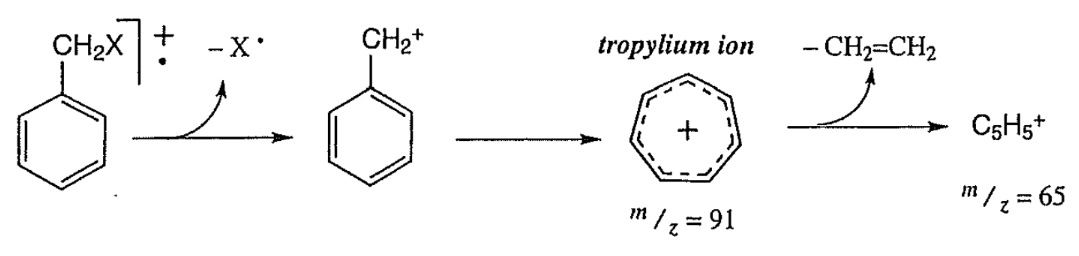
- The most likely fragmentation occurs at the weakest C-C bond.
- Typically, only one break is made per ionization event.
-
Example - Benzene Fragmentation:
Isotope Patterns
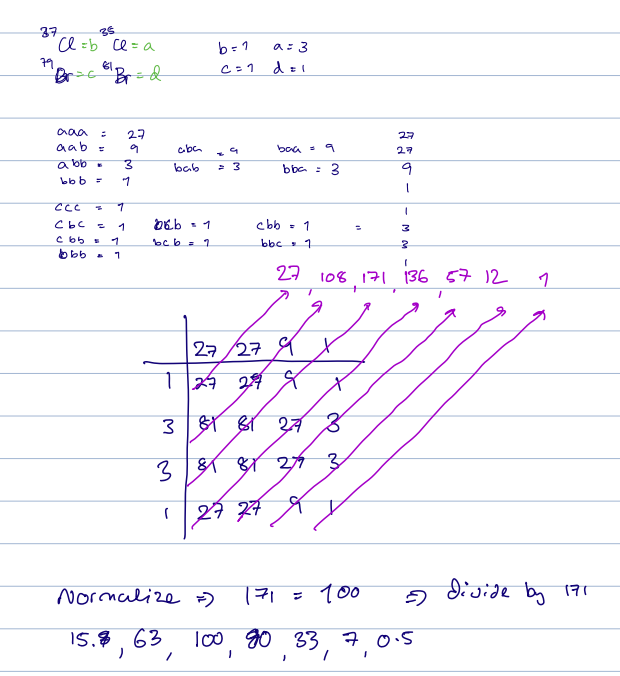
- Calculating Isotope Patterns:
- Isotope peaks can reveal the elements present in a molecule based on their specific patterns.
- For example, the isotope pattern for Cl
Br : - Elements like Br and Cl create distinctive patterns:
- Br: 79/81 amu with 50% abundance each
- Cl: 35/37 amu with 75%/25% abundance
How to Approach a Mass Spectrum
-
Molecular Ion Peak:
- Identify the highest mass peak (excluding isotopes).
- In EI ionization, the M
ion is observed, whereas softer methods show MH or M(NH ) , etc. - Apply the Nitrogen Rule:
- If the MI peak is even, the number of nitrogen atoms (N) is 0, 2, 4, etc.
- If the MI peak is odd, N is 1, 3, 5, etc.
- Mass to structure calculator
-
Isotope Peaks:
- Analyze isotope peak patterns to determine elements.
- Check mass differences and relative intensities.
- For example, the isotope pattern of 2 Br in a molecule results in a 1:2:1 ratio.
-
Number of Rings and Double Bonds:
- Calculate using the formula:
- Rings + Double Bonds
for
- Rings + Double Bonds
- Calculate using the formula:
-
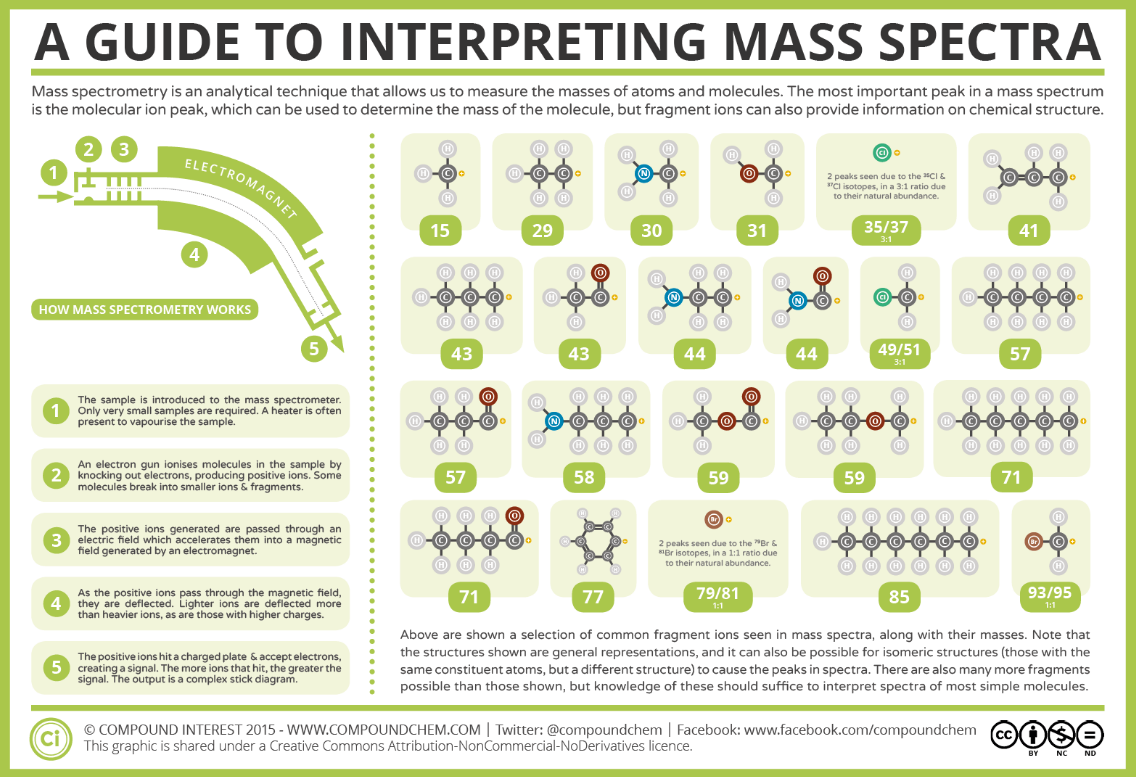
Common Fragmentation Patterns:
Resources
- Books and Links:
- Great overview chapter: Introduction to Organic Spectroscopy
- Mass to structure calculator