- Wavelength: 190 - 900 nm
- Measures: Electronic emission
- Uses: conjugation Aromatic Concentration
- General notes
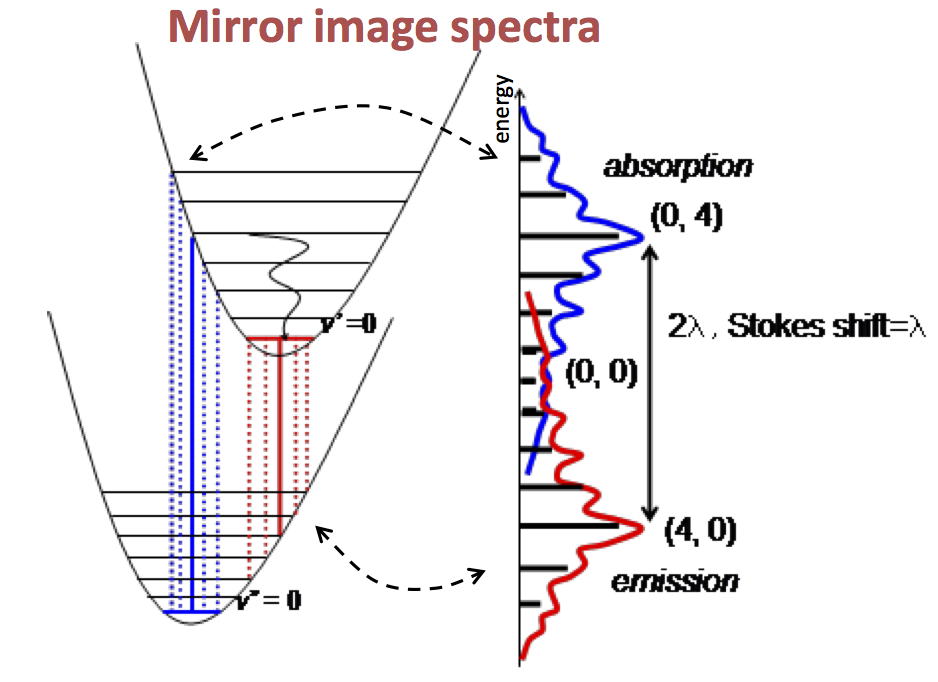
- Absorption and emission spectra are usually mirrored.
- Due to Kasha's Rule, all observed transitions are between S1, S0, and T1 states.
- A fine structure is observed from the relaxation to higher vibrational states of S0.
- The most intense transitions occur at the highest orbital overlap, according to the Frank Condon principle.
- If orbitals are shifted and there is imperfect overlap, the 0,0 transition may lose intensity or disappear, resulting in a Stokes Shift.
- Transitions are numbered in the form ((\nu,\nu^{\prime})), and depending on the overlap of wavefunctions, the major peak may vary.
-
Kinetics
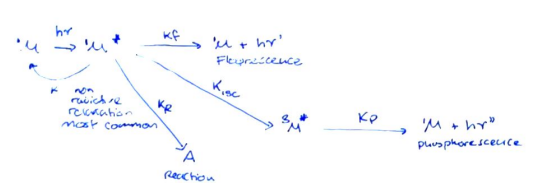
- There are multiple competing processes that govern the kinetics.
- Quantum yield (\phi = \text{Quantum yield} = \frac{\text{No. } M^* \text{ that give } h\nu}{\text{No. } M^* \text{ made}} = \frac{k_r}{k_{tot}})
- The higher the quantum yield, the better the spectral imaging.
- If a molecule cannot do ISC, (\phi = \frac{k_{rad}}{k_{nr}+k_{rad}}), where (nr) denotes non-radiative processes.
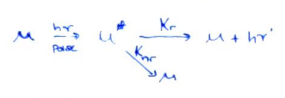
- Lifetime (\tau) is the duration for which (M^*) exists: (\tau = \frac{1}{k_{tot}}).
- (\frac{d[M^]}{dt} = -k_{rad}[M^]-k_{nr}[M^*])
- (\frac{d[M^]}{[M^]} = -(k_{rad}+k_{nr})dt)
- (M^* = [M^*]_{(t=0)}\exp{(-t/\tau)})
- Fluorescent: (\tau) = 10 ps - 100 ns
- Phosphorescent: (\tau) = 100 ns - 1 s
-
- Excitation is achieved using a sharp, short-pulsed laser.
- After each pulse, emission is measured with respect to time.
- The shorter the pulse, the better the spectrum. Poor pulses can be deconvoluted.
-
Quenching
- A Quencher can accept the excitation from a molecule and let it relax without light formation.
- (M^* + Q \rightarrow Q^* + M \rightarrow Q + M)
- The more Q is added, the less emission is measured.
- (\frac{d[M^]}{dt} = -(k_{nr}+k_{rad})[M^]-k_q[Q][M^*])
- Spectra can be compared with and without quencher analysis via a Stern-Volmer analysis specdef.
- (\frac{I_f^o}{I_f} = \frac{\phi_f^o}{\phi_f} = \left(\frac{k_{rad}}{k_{nr}+k_{rad}}\right)\left(\frac{k_{rad}}{k_{nr}+k_{rad}+k_{Q}}\right)^{-1} = 1 + \tau_0 k_q [Q])
- A Stern-Volmer plot quantifies fluorescence loss as a function of quencher addition specdef.
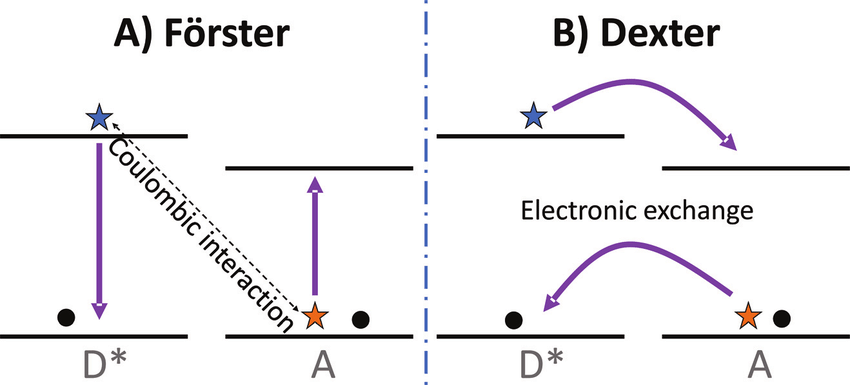
- Quenching mechanisms
- (^{1}M^* + Q \rightarrow \ ^{1}M + Q^*)
- (^{3}M^* + \ ^{1}Q \rightarrow \ ^{1} M + \ ^{3}Q^*)
- (^{3}M^* + \ ^{3}Q \rightarrow \ ^{1} M + \ ^{1}Q^*)
- Use of the Stern-Volmer plot in real life:
- A polymerization is carried out with a chromophore and quencher.
- In the liquid state, quenching occurs easily.
- In the solid state, quenching can no longer occur, so fluorescence increases.
-
Polarization
- The transition dipole moment results in polarized light being emitted, but tumbling molecules mean this cannot be measured.
- To measure polarization, the sample must be frozen as a glass.
- Anisotropy is the extent of polarization of light specdef.
- This can be plotted over a reaction showing when solidification takes place, e.g., resin curing.
-
Instrumentation #UV-vis
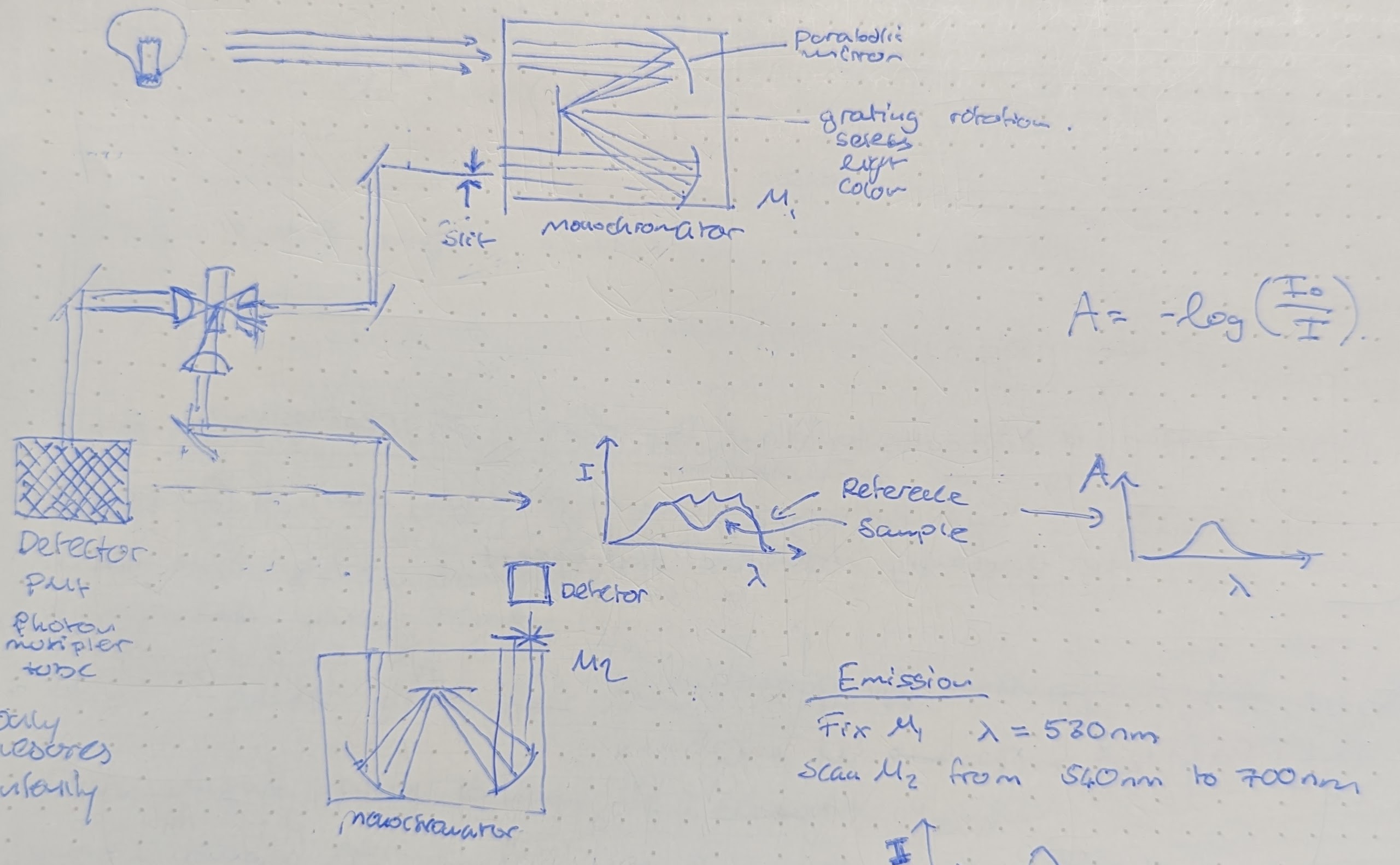
- Emission scan - Fixed incident wavelength, emission wavelength scanned specdef.
- Excitation scan - Scanned incident wavelength, fixed emission detection specdef.
- Can pick up the emissive parts of absorption experiments.
- Inner filter effect - Highly concentrated samples may result in lower emissions due to all excitation happening at the light source point and not through the sample.
- Raman shifts may be visible in very low concentration spectra.
- Flashcards
- How is quantum yield calculated? spcards
- (\phi = \frac{No. \ M^* \ that \ give \ h\nu}{No. \ M^* \ made} = \frac{k_f}{k_{tot}})
- (\phi = \frac{k_{rad}}{k_{rad}+ k_{non \ rad}})
- How is the lifetime (\tau) of an excited molecule calculated? spcards
- (\tau = \frac{1}{k_{tot}})
- What is the typical lifetime of a fluorescent compound? spcards
- What is the lifetime of a phosphorescent compound? spcards
- In Time Resolved Emission Spectroscopy (TRES), what kind of light source is used? spcards
- Sharp, short-pulsed laser.
- How can badly pulsed spectra be improved in emission spectroscopy? spcards
- What is a Stern-Volmer plot? spcards
- (\frac{I_f^0}{I_f} = \frac{\phi_f^0}{\phi_f} = 1 + \tau_0 k_q [Q])
- Fluorescence as a function of quencher addition.
- Give the Forster Mechanism for quenching. spcards
- (^{3}M^* + \ ^{1}Q \rightarrow \ ^{1} M + \ ^{3}Q^*)
- Give the Dexter Mechanism for quenching. spcards
- (^{3}M^* + \ ^{3}Q \rightarrow \ ^{1} M + \ ^{1}Q^*)
- Why can we not measure polarization in liquid state emission spectroscopy? spcards
- The molecules are tumbling.
- What is Anisotropy? spcards
- The extent of light polarization.
- What is an Emission scan? spcards
- Fixed incident wavelength, emission wavelength scanned.
- What is an Excitation scan? spcards
- Incident wavelength scanned with fixed emission wavelength detection.
- What is the Inner filter effect? spcards
- Highly concentrated samples show low emission due to emission occurring close to the light source and not through the whole sample.