Summary
Electron Paramagnetic Resonance (EPR), also known as Electron Spin Resonance (ESR), is a spectroscopic technique used to detect and study chemical species with unpaired electrons, such as radicals, transition metal complexes, and defects in materials. EPR provides insights into the electronic structure, local environment, and dynamics of these paramagnetic species.
Experiment
- Paramagnetic sample prepared
- Avoid too high concentrations, otherwise there will be spin-spin T2 relaxation
- A Uniform magnetic field is applied to split energy levels by the Zeeman Effect
- Irradiation with microwaves of a fixed frequency (standing wave in the cavity) while the magnetic field is swept.
- Resonance occurs when the microwave energy matches that of the Zeeman splitting.
energy difference between spin states
Electron g-factor
Bohr magnetron
Magnetic field strength
- Absorption is detected by detector and plotted as a derivative spectrum
Theory
Basis
- Zeeman Effect In a magnetic field, the energy of an electron’s spin state splits into two levels:
where - Therefore
- Therefore
- Absorption of the microwaves occurs when the photon radiation matches the energy gap:
Microwave frequency
- Unpaired electrons interact with nearby magnetic nuclei causing Hyperfine Splitting of the EPR signal resulting in coupling constant
. - Selection Rules
- The spin hamiltonian
- Describes the energy of a paramagnetic system according to interactions that depend on the orientation of the magnetic field relative to the magnetic frame.
: Bohr magneton. : External magnetic field vector. : g-tensor (3×3 matrix) describing the anisotropy in the Zeeman interaction. : Electron spin operator vector. : Zero-field splitting (ZFS) tensor, significant for systems with : Nuclear spin operator vector. : Hyperfine coupling tensor, representing the interaction between electron and nuclear spins. : Nuclear quadrupole coupling tensor, relevant for nuclei with spin .
- This is comprised of a few different terms.
- Describes the energy of a paramagnetic system according to interactions that depend on the orientation of the magnetic field relative to the magnetic frame.
Hyperfine Splitting
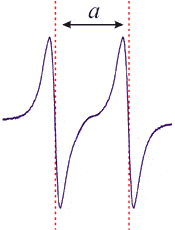
= number of peaks where is the # nuclei and the nuclear spin - The Hydrogen radical
has the following splitting: 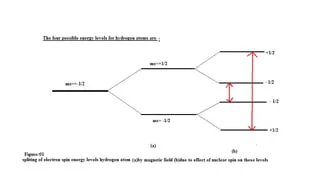
- Therefore this spectrum
- Eg. V4+ I= 7/2
- Produces 8 equal intensity peaks
- Isotropic phenomenon
- Fermi Contact Interaction
- S-electron density
- Anisotropy
- Dipolar effects
- Electron/nuclear movement
- Zero-field Splitting
- Happens in some octahedral/Tetrahedral complexes where even at
there is still splitting of states resulting in a Kramer's Doublet
- Happens in some octahedral/Tetrahedral complexes where even at
Solid-State EPR (SSEPR)
- In the Anisotropic case, we no longer have averaging for the spin hamiltonian. Therefore, the electron spin orerator vector and g-tensor now are no longer averaged.
- Isotropic
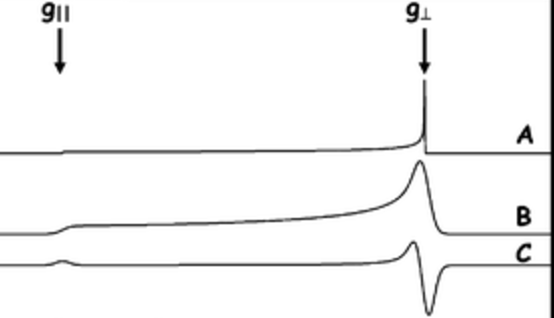
- Axial Case
- Therefore we have vertical and horizontal components where the vertical is half as tall as the horizontal.
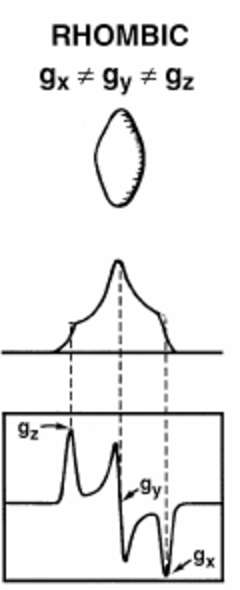
- Rhombic case
- Overlap of three peaks
Sensitivity
- Higher energy gap = better sensetivity = lower signal intensity
- At lower temperatures there is a bigger boltzmann population difference which aids the EPR effect
- When there is saturation
there is no signal.
- We know the Zeeman effect is
- A free electron has
which is the total magnetic dipole moment. - Transition metal complexes have large variations in
-factor. - Organic Radicals have
-factors glose to - Spin-orbit angular momentum coupling has the largest influence on the
-factor - Heavy atom localizedd radicals have low g-factor, light atom bound or delocalized electrons have higher g-factor.
Relaxation
- spin-lattice relaxation
(most important at r.t.) - Spin system with surrounding molecules, energy dissipation via vib. rot. trans.
time to lose in energy.
- spin-spin relaxation
- Relaxation between excited spin electron and nearby electron or nucleus.
- Larger at high concentration
- resonance line-width determined by relaxation times
- Heisenberg Spin Exchange - Tumbling molecules collide therefore switching spins.